What is Nrf2?
Our DNA encodes about 20,000 genes, each representing a “blueprint” for the production of a protein or enzyme necessary for a healthy existence. Each of these “blueprints” requires a regulating control called a “promoter” that determines precisely how much of each product is produced, and under what circumstances. By binding to one specific type of these switch-like promoter regions called the “Antioxidant Response Element (ARE)” the Nrf2 factor controls the rate of production from hundreds of different genes that allow cells to survive under stressful conditions. These genes produce a large variety of antioxidant enzymes that create a network of protection by neutralizing primary and secondarily generated oxidants and by cleaning up the toxic byproducts they leave in their wake, as well as by helping to repair the damage they have caused.
What is oxidative stress?
Oxidants such as the superoxide radical (O2-.) and hydrogen peroxide (H2O2) are produced by the process of “burning” the foods that sustain us, just as the internal combustion engines in our cars produce similar reactive chemicals. Cars have catalytic converters to neutralize and detoxify these reactive chemicals; our bodies have antioxidant enzymes to accomplish the same result. The Nrf2 pathway senses the need for these antioxidant enzymes and regulates their production to maintain metabolic balance. Several things can upset this delicate balance, and simply growing older is one of them. We produce less Nrf2 as we age, and the balance slowly tips toward the oxidative side—a condition known as “oxidative stress.” Disease processes can also result in overproduction of oxidants. Infections, allergies, and autoimmune diseases activate our immune cells, which produce reactive oxidants (O2-., H2O2, OH. and HOCl) in order to kill germs that the immune system assumes to be present, but our otherwise healthy cells get caught in the cross-fire and sustain collateral damage that we see and feel as inflammation. Other major diseases associated with aging, such as heart attacks, stroke, cancer, and neurodegenerative conditions such as Alzheimer’s disease also increase production of oxidants, creating oxidative stress, and inflammation.
What are Nrf2 activators?
The Nrf2 protein, known as a transcription factor because of its ability to control genes, is the key component of a pathway (a sequence of biochemical reactions in a cell) that senses and responds to changes in oxidative balance. The sensing components of the pathway chemically modify and release Nrf2 (i.e., they activate it) so that it may diffuse into the nucleus of the cell where the DNA resides. It can then “switch on” or “turn off” the genes it controls (often termed survival genes) to produce the protected state within the cell. Fortunately, many of the chemical compounds that are Nrf2 activators are produced by certain plants, crude extracts of which were known to our ancestors centuries ago in traditional Chinese, Ayurveda, and Native American medicines. These phytochemicals appear to be just as potent, and to have fewer (if any) side-effects, as the Nrf2-activating pharmaceutical products that are beginning to appear.
How has the science behind Nrf2 activation advanced in the last decade?
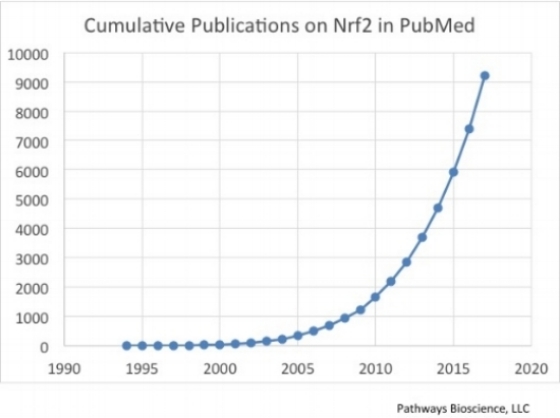
In 2004, when the first successful Nrf2-activating dietary supplement was being developed, very little was understood regarding the Nrf2 pathway. There were about 200 papers in the literature on Nrf2 (NFE2L2) and scientists were just beginning to paint the picture of Nrf2 as the “master regulator” of the antioxidant response in mammals. As of 2017, more than 9,300 academic studies have been published, and the title of “master regulator” has proven to be well-deserved. Nrf2, in fact, regulates many hundreds of genes that have nothing to do antioxidant enzymes per se, but rather provide protection from a broader range of stress-related events that are encountered by cells, organs, and organisms, under both normal and pathological circumstances. Obviously, we are now in a much better position to redesign, from the ground up, a new family of Nrf2 activating dietary supplements and potential therapeutic agents, based on this massive amount of information learned from published academic research, as well as from our own proprietary research, than we were in 2004.
As recently as 2007 we described the Nrf2 activation in rather simplistic terms—pretty much a one-step process of activating a specific kinase that phosphorylated Nrf2, releasing it to enter the nucleus. The years since then have produced thousands of studies shedding much more light on the intricacies of the pathway. Of great importance to us is that a fuller understanding reveals many more opportunities for optimizing control and regulation of the pathway. Many of the newly discovered steps in the pathway come as no surprise as they mimic structures and regulatory points of numerous other pathways found in our bodies. For example, an automobile would be of little practical use if it provided a single control in the form of an accelerator. Without brakes or steering, it would be impossible to use under virtually all desired applications. So it is with biochemical pathways—they must be responsive to changing needs and able to sense changing conditions to maintain proper balance. Our current working view of the Nrf2 pathway is much more complicated than was appreciated a decade ago. Using this knowledge to our advantage we have developed what we call Nrf2.0® Technology.
Why do we need Nrf2-activating supplements or therapeutics as we age?
Why should we be concerned with supplements or therapeutics that can adjust the Nrf2 pathway? Isn’t it, after all, part of a very large network of interactive and self-regulating pathways that automatically adjust to achieve proper balance based upon our metabolism? In a young healthy animal, this appears to be the case. In post-reproductive organisms, however, things begin to change, as Nrf2-activation capabilities decline with age. As harsh as it may sound, after animals have passed on their DNA and reared their offspring they have mostly served their purpose as far as Mother Nature is concerned While it may not be nice to fool Mother Nature, perhaps people can agree that it’s now acceptable to tinker a little with Mother Nature.
Many studies have shown that the concentration of Nrf2 in cells declines as animals age. Old age brings with it increased markers of oxidative stress and an assortment of “age-related diseases” such as atherosclerosis and cardiovascular disease, cancer, arthritis, cataracts, osteoporosis, type 2 diabetes, hypertension and Alzheimer's and Parkinson’s diseases. All of these diseases have been characterized by evidence of increased oxidative stress. By gently stimulating our aging cells’ ability to activate and modulate Nrf2, we aim to restore and enhance our body’s own ability to counteract the effects of oxidative stress.
In our experience with human subjects, the level of plasma TBARS (thiobarbituric acid)—which is a measure of oxidative stress—have all declined to the Target Range of 1.0-1.5 μM (μM malondialdehyde equivalents) within a span of about a month.
Nine human subjects with elevated plasma TBARS (greater than 1.5 μM) are shown here, before and after supplementation with PB125. In all cases TBARS dropped to below the target level of 1.5 μM.
Polyunsaturated fatty acids (PUFAs) are among the most easily oxidized molecules in our bodies, and they are especially vulnerable to attack by free radicals. Their oxidation produces TBARS, which are widely used as a sensitive measure of oxidative stress. The oxidation of PUFAs is why dead fish begin to smell bad very quickly! It is also why omega-3 fatty acid supplements may start out as fish oil but can quickly oxidize and turn into TBARS supplements.
TBARS in your body gradually increase as you age, indicating a decrease in Nrf2-regulated pathways and a rise of oxidative stress.
What is Nrf2.0® Technology?
Based on the extensive amount of new, published scientific literature and our own research we have identified at least five control points in Nrf2 activation in which the magnitude, duration, and quality of a Nrf2-activating event may be modified. Perhaps the single most significant new area is the “shutdown” part of Nrf2 activation; taking the foot partially off of this brake that is otherwise halting Nrf2 activation allows the Nrf2 that is activated by dietary signals to remain in the nucleus a little longer and induce more expression of protective genes. Most biochemical pathways, in fact, have this type of “on” and “off” pathways running concurrently, but at different rates, in order to provide quick response time. Formula One race car drivers entering tight curves must perfect a “heel-and-toe” maneuver in which the right foot heel is on the accelerator while the toe is on the brake (and the left foot is on the clutch), all in the interest of the quickest performance. Biologically, gene induction is a very slow mechanism (requiring hours) for controlling flux through a pathway; therefore, many enzymes have their own on/off switches involving phosphorylation or other covalent modifications that can be activated in minutes by various regulatory enzymes.
Pathways Bioscience has developed proprietary, patent-pending compositions that make use of this expanding knowledge base of Nrf2 activation. We now know that Nrf2 activation consists not only of the Nrf2 transcription factor being released from its inhibitor, migrating into the cell nucleus, and binding to specific DNA sequences that promote cytoprotective gene expression, but also on modulating the rate at which Nrf2 is removed from the nucleus. Expanded understanding of the Nrf2 activation and removal process enabled us to develop combinations of dietary agents to achieve improved expression of cytoprotective genes via its modulation. The combination of this enhanced knowledge base, along with our own proprietary research, helped us create the second generation of Nrf2 activators for use as dietary supplements. This forms the foundation of our Nrf2.0® Technology family of products.